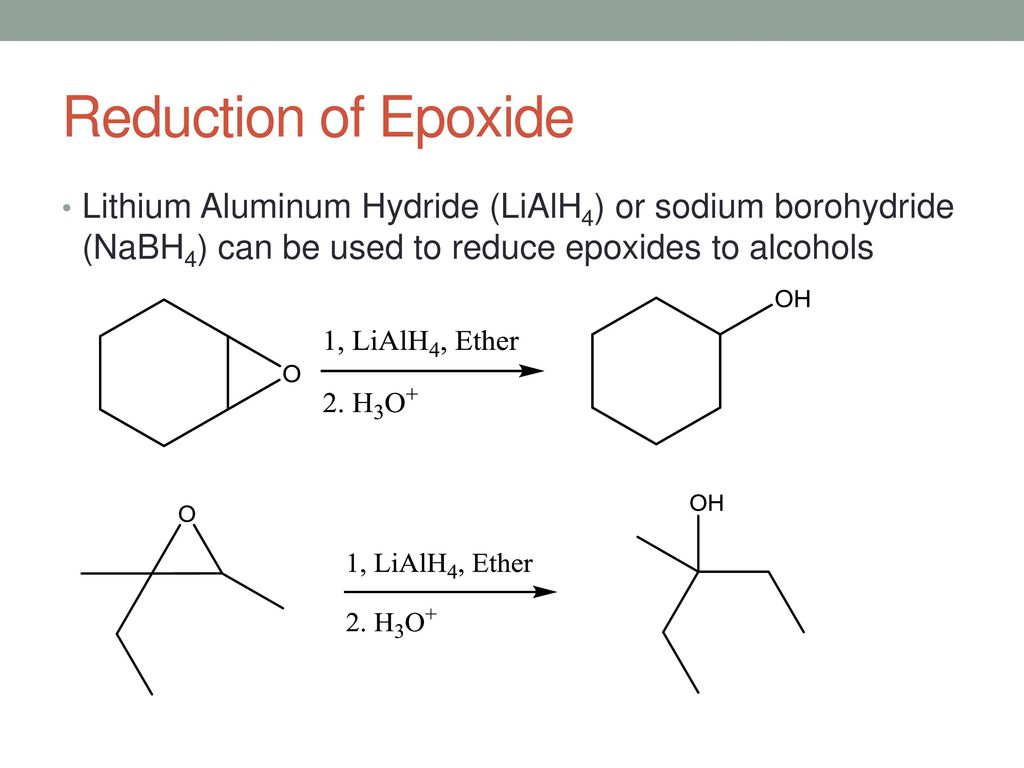
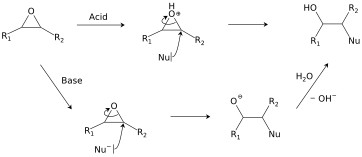
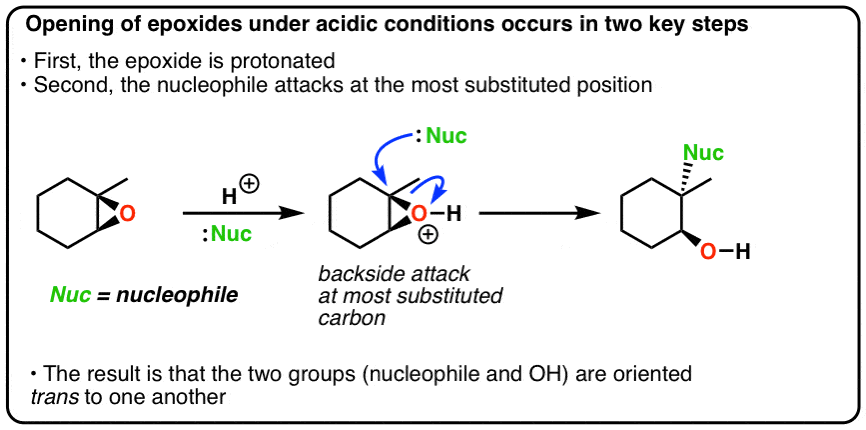
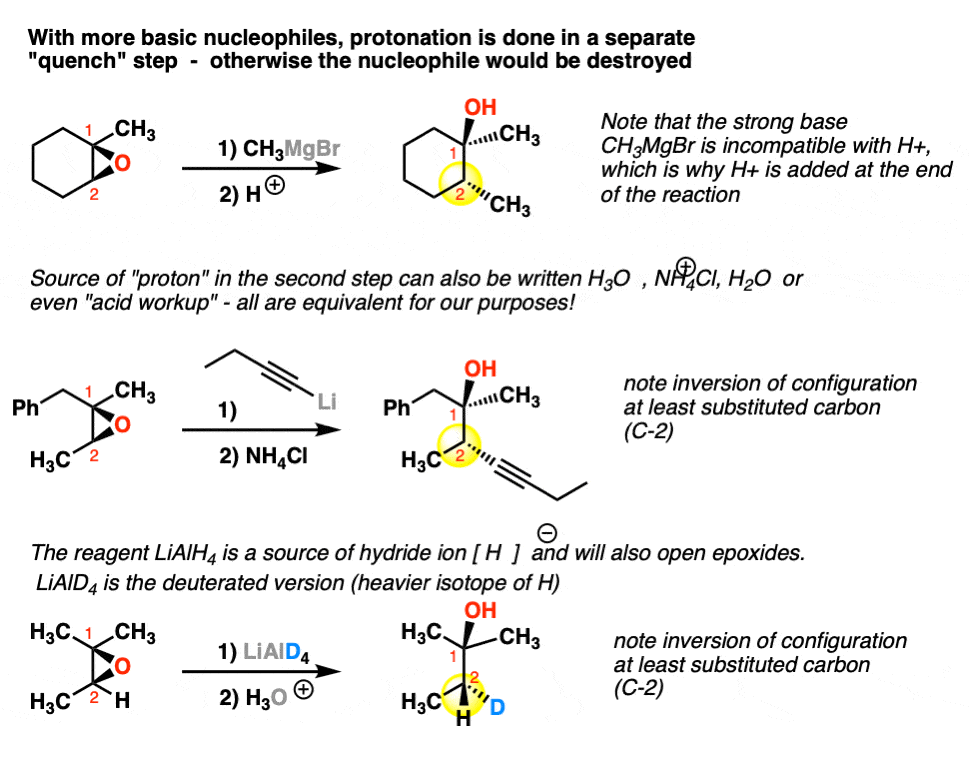
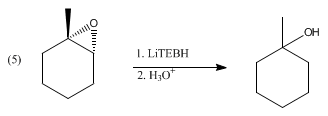Catalytic Reduction of Cyclic Ethers with Hydrosilanes - Park - 2019 - Chemistry – An Asian Journal - Wiley Online Library
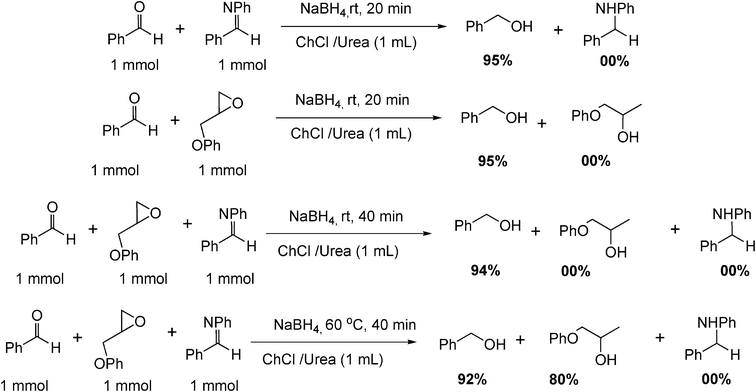
Natural deep eutectic salt promoted regioselective reduction of epoxides and carbonyl compounds - RSC Advances (RSC Publishing) DOI:10.1039/C2RA01280D
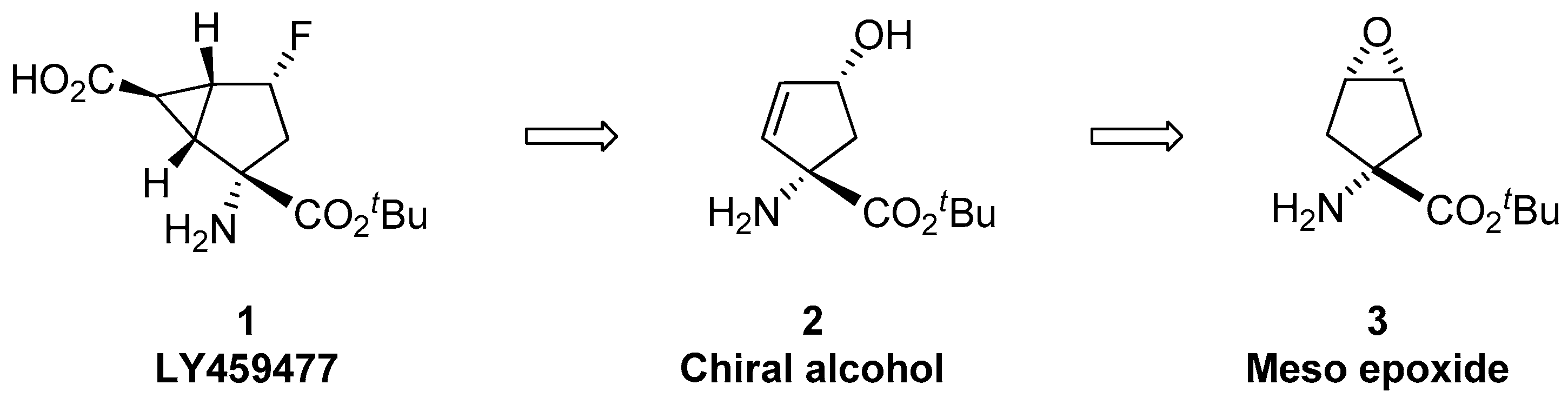
Recent applications of Cp 2 TiCl in natural product synthesis - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C3QO00024A

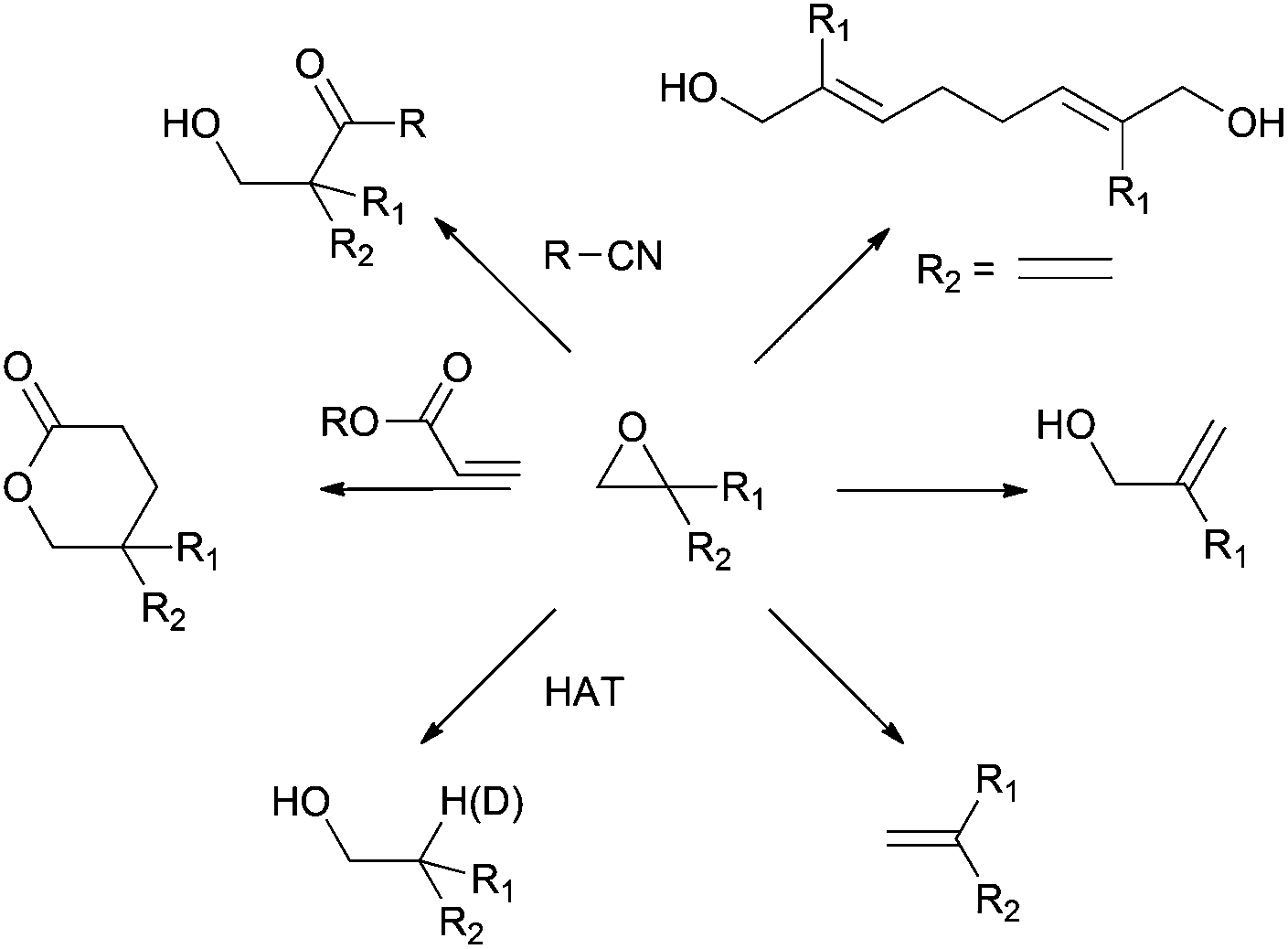
Catalytic reductive ring opening of epoxides enabled by zirconocene and photoredox catalysis - ScienceDirect

Catalytic Hydrogenation of Epoxides to Alcohols - Thiyagarajan - 2022 - Chemistry – An Asian Journal - Wiley Online Library

Synthesis of α-Alkylated Ketones via Selective Epoxide Opening/Alkylation Reactions with Primary Alcohols | Organic Letters
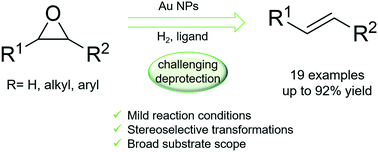
Clean protocol for deoxygenation of epoxides to alkenes via catalytic hydrogenation using gold - Catalysis Science & Technology (RSC Publishing)

A New and Efficient Epoxide Ring Opening via Poor Nucleophiles: Indole, p-Nitroaniline, Borane and O-Trimethylsilylhydroxylamine in Lithium Perchlorate